Spot urine protein excretion during the first year after kidney transplantation associates with allograft rejection phenotype at 1-year surveillance biopsies: An observational national-cohort study
Miha Arnol1,2, Manca Oblak1, Gregor Mlinšek1, Aljoša Kandus1, Maja Frelih3, Nika Kojc3.
1Department of Nephrology, Centre for Kidney Transplantation, University Medical Centre Ljubljana, Ljubljana, Slovenia; 2Department of Internal Medicine, Medical Faculty, University of Ljubljana, Ljubljana, Slovenia; 3Institute of Pathology, Medical Faculty, University of Ljubljana, Ljubljana, Slovenia
Introduction: Urine protein excretion is routinely measured to assess kidney allograft injury, but the diagnostic value of this measurement for kidney transplant pathology remains unclear. Here we investigated whether spot urine protein excretion during the first year after kidney transplantation associates with allograft rejection phenotype and Banff histopathologic scores at 1-year surveillance biopsies, and de-novo occurrence of donor-specific antibodies (DSA).
Patients and Methods: This prospective, observational, national-cohort study included 139 consecutive non-sensitized patients who received a deceased donor kidney transplant between Dec 2013 and Dec 2017. All patients received basiliximab induction and tacrolimus-based immunosuppression. Estimated protein excretion rate (ePER) was calculated monthly from spot urine protein-to-creatinine ratios. At 1 year, all recipients underwent surveillance graft biopsy and were screened for de-novo DSA. Screening-positive sera were subjected to single antigen bead (SAB) testing (LABScreen SAB assays; One Lambda). The occurrence of de-novo DSA was determined on the basis of SAB reactivity patterns using a mean fluorescence intensity threshold >1000. All biopsies were independently read by two pathologists and were graded in accordance with Banff 2013 criteria.
Results: Among the 139 study patients, 27 patients (19%) had histologic evidence of T-cell mediated rejection (TCMR; 8 patients borderline Banff 3, 10 patients tubulointerstitial Banff 4/1, and 9 patients vascular Banff 4/2), and 9 patients (7%) had histologic evidence of antibody-mediated rejection (ABMR) at 1-year surveillance biopsy. One year after transplant, 19 patients (14%) developed de-novo DSA (4 patients class I, and 15 patients class II). Greater levels of ePER at 1 year were significantly associated with Banff histopathologic scores and greater incidences of ABMR, vascular TCMR rejection, and de-novo DSA (Table).
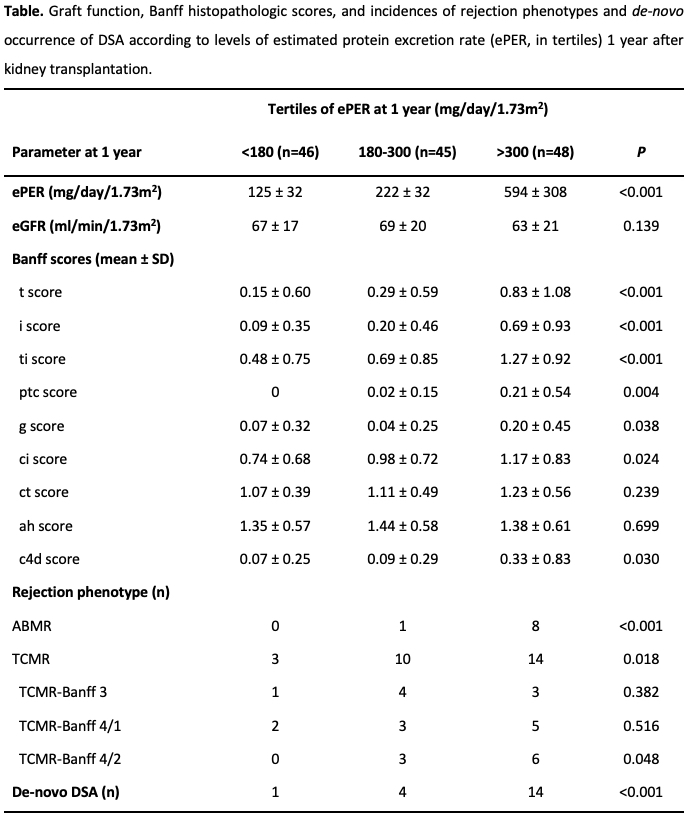
Compared with non-rejection findings, TCMR, and no de-novo DSA, the linear mixed effect regression analysis demonstrated a progressive increase and a significant difference in slope of ePER during the first year among patients with ABMR and de-novo DSA at 1 year (P<0.001) (Figure 1).
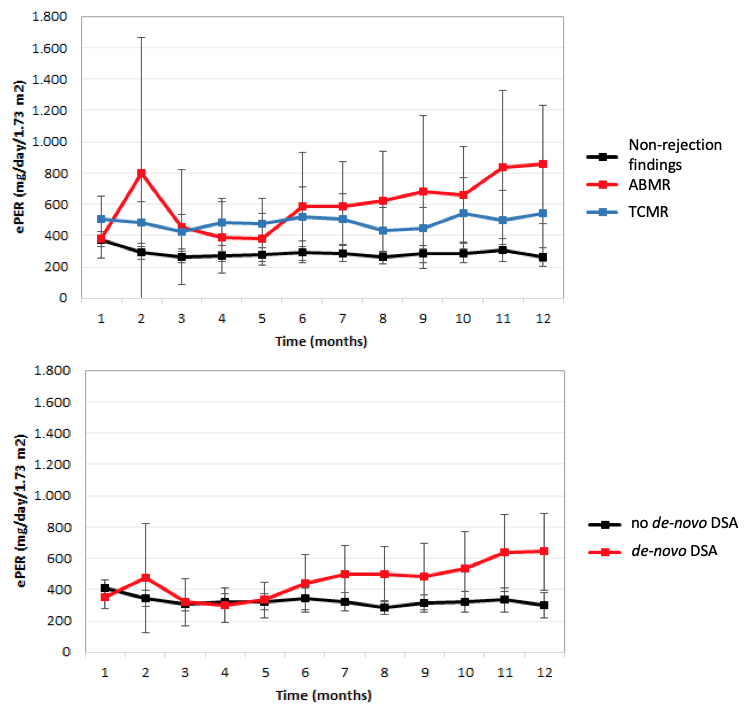
Patients with vascular TCMR-Banff 4/2 also demonstrated a significant increase in slope of ePER over time when compared with patients with other TCMR phenotypes (P=0.014) (Figure 2).
The discriminatory power of ePER for intragraft rejection processes was better in patients with ABMR (AUC 0.95, 95%CI 0.90–0.99; P<0.001) than in those with TCMR (AUC 0.68, 95%CI 0.59–0.79; P=0.002), with 89% sensitivity and 93% specificity for proteinuria >550 mg/day/1.73m2.
Conclusions: An increase in low-grade ePER during the first year after kidney transplantation associates with ABMR, vascular TCMR, and de-novo DSA at 1-year and may be used as a non-invasive clinical marker of intragraft endothelial cell injury.
There are no comments yet...